Vaccines based on virus-like particles and mRNA
For viral infections the number of antiviral drugs is relatively low, and vaccines remain the main factor of protection. Therefore, the development of new vaccines or improvement of already existing antiviral vaccines is of paramount importance. Historically two types of vaccines were used against viral diseases – killed virus vaccines or attenuated viral strains. With the advent of recombinant era technologies, a number of other approaches came into light, namely vaccines based on recombinant viral proteins, viral DNA vectors or viral RNAs. Out of recombinant protein vaccines, the most important ones that found application in human therapy in the last two decades are so called virus-like particles. They can be defined as empty shells of viruses resembling authentic viruses but devoid of genetic material, so the virus cannot multiply. However, virus-like particles retain many properties of viruses – they can enter cells using the same receptors as viruses and they provoke very strong immunological response of both types important for conferring protection: humoral and cellular response. As the present pandemic shows another type of antiviral vaccines, RNA vaccines, are extremely effective. In contrast to DNA, RNA is not integrated into cell genomic material, so host mutations resulting from the administration of these vaccines are not possible.
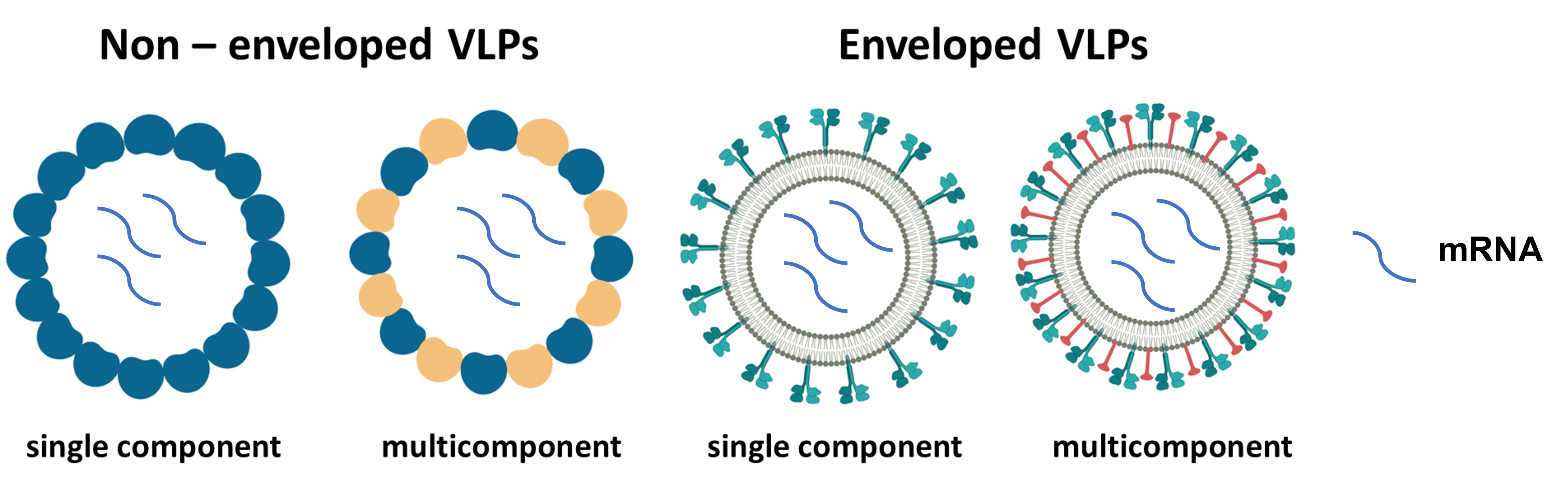
Moreover, the cost of production of RNA vaccines is relatively low. The main drawbacks of RNA vaccines are the difficulties in the delivery to the interior of cells and their instability when they enter cells; these factors hinder their long-time effect. To overcome the drawbacks of RNA vaccines and to augment their immunological properties we plan to produce vaccines on the basis of combined superstructures containing both virus-like particles and mRNA. Our studies, in collaboration of Prof. Jacek Jemielity of the University of Warsaw, are supported by Polish National Science Centre (NCN) under the grant “Mammalian cell delivery of stable therapeutic mRNA packaged inside virus-like particles”. Our prospective aim is to produce novel recombinant vaccines against two devastating diseases: influenza and tick-borne encephalitis. Seasonal influenza affects each year over 10% of world population, while the incidence of tick-borne encephalitis has increased over 400% during the past 20 years in Europe. The existing vaccines against these diseases are far from being perfect. Influenza vaccinations have to be repeated every year and the effectiveness of protection rarely exceeds 50%; tick-borne encephalitis vaccine can cause adverse reactions, especially in children. Our project application addresses the needs for vaccine improvements as our prospective aim is to obtain effective universal vaccine against influenza with long-lasting protection and anti-tick-borne encephalitis vaccine with no side effects.



