The mechanisms of virus immune evasion, virus-induced protein degradation, interactions of viruses with extracellular vesicles, and modulation of autophagy
We study various strategies developed and highjacked by viruses to avoid recognition by the immune system of the host. Structural and protein modeling studies (especially in membrane-mimicking environment, in collaboration with the Faculty of Chemistry) followed by side-directed mutagenesis are employed to elucidate mechanisms of activity of UL49.5 protein, the main immunomodulatory protein in some herpesviruses, which downregulates major histocompatibility class I (MHC I).
Using fluorescently labeled proteins (like the TAP transporter – the cellular target of UL49.5) and genome-wide siRNA/CRISPR-Cas9 screens, we search for cellular proteins involved in the virus-induced degradation, studying mechanisms of ERAD (ER-associated protein degradation) and the role of degrons in viral proteins.
The methodology developed for our research on herpesviruses is also used to study the immunomodulatory properties of SARS-CoV-2 and other viruses (like TBEV – tick-borne encephalitis virus).
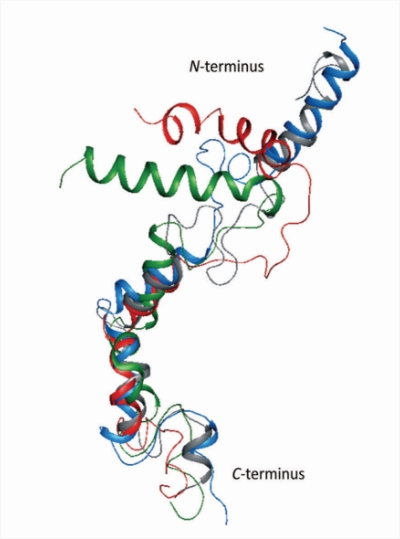
UL49.5 and its variants – a search for structure-function correlations
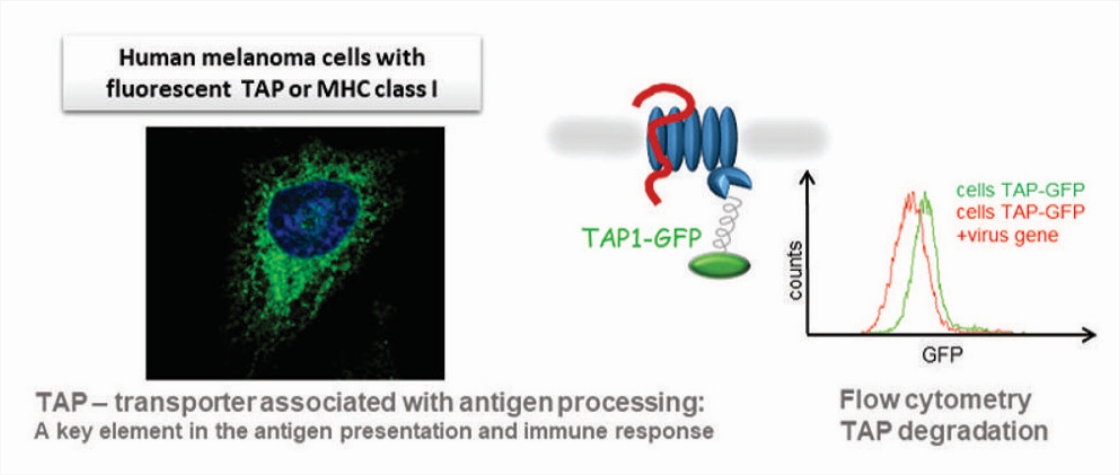
A pipeline to study the immunomodulatory function of virus proteins
We study interactions of viruses with cell-produced extracellular vesicles (EVs): which viral proteins are loaded to EVs, which microRNA can be found as EVs cargo, and what are the consequences of EVs internalization.
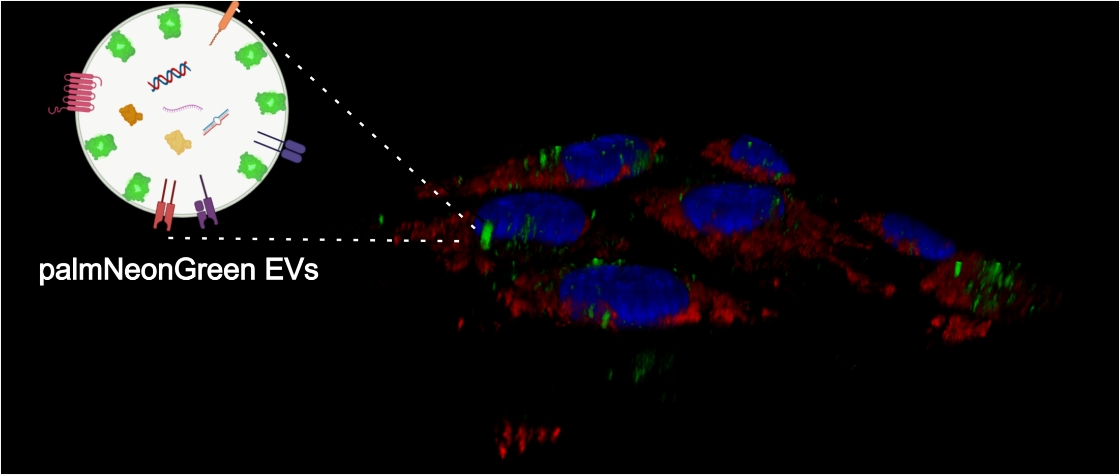
Cells internalizing fluorescently-labeled EVs with virus cargo.
Our research also focuses on the modulation of cellular autophagy pathways (natural cellular processes to keep cell fitness) by herpesvirus and coronavirus proteins.
Our aim is to contribute to the understanding of the pathogenesis of viruses and identify targets for future therapies and vaccines.



