Molecular mechanisms of herpesvirus latency and contribution of viral infection to oncogenesis
We investigate the molecular mechanisms of herpesvirus latency and the potential role of virus in oncogenesis and/or oncostimulation. Infection with human cytomegalovirus (HCMV) is very common. Primary HCMV infection in healthy individuals is usually asymptomatic, however in immunocompromised patients, for example after transplantation or with AIDS, it may cause severe disease. HCMV is also a cause of congenital disease, because this virus is able to cross from the blood circulation of the mother, through the placenta to the fetus. HCMV congenital disease is associated with infection of the central nervous system and consequently neural developmental disabilities. Symptoms associated with the HCMV congenital disease are for example: hearing loss, eye sight compromise, and learning disabilities.
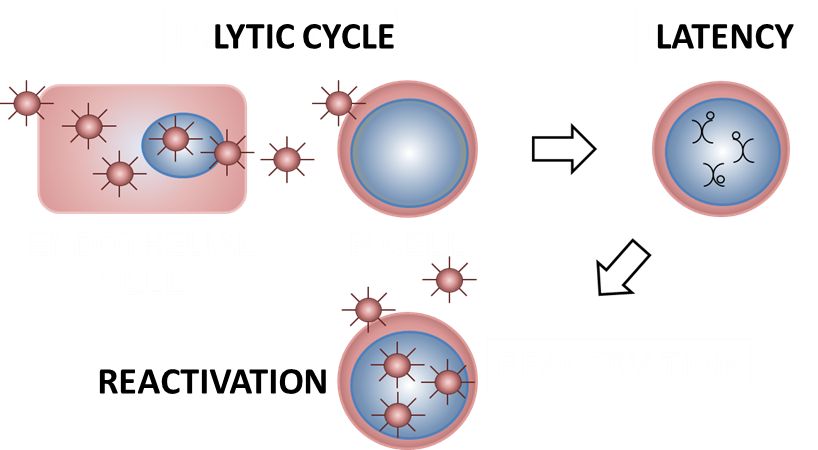
As is the case for all the herpesviruses the life cycle of HCMV can be divided into lytic phase, when the virus is replicating, and the latent phase, in which virus persists in the cell. Part of our research focuses on molecular aspects of HCMV latency. In the case of HCMV the viral protein responsible for latency maintenance is not known yet. In glioblastoma cells we detected a novel localization pattern of HCMV IE1 protein on mitotic chromosomes, which seems to be specific to this type of cancer cells. IE1 was previously shown to localize on mitotic chromosomes evenly covering them in a pattern called: ”chromosome painting”. In glioblastoma cells we observe a novel localization of IE1 to chromosome-associated spots (CAS). IE1 is one of the HCMV proteins found in high percentage of glioblastomas.
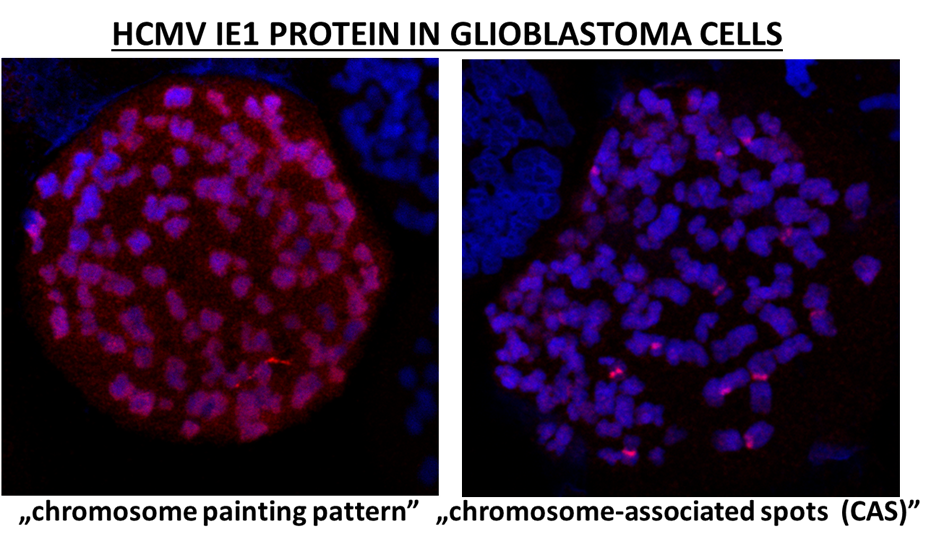
Glioblastoma multiforme (GBM) is a malignant brain tumor with a very poor prognosis for the patients. The etiology and pathogenesis of this disease are still not understood, which hinders development of successful therapies. High percentage of glioblastomas were found to be positive for human cytomegalovirus (HCMV), which was shown to be associated with worse prognosis for the patients. Anti-HCMV treatments have shown increase in survival rates for GBM patients, confirming influence of HCMV infection on the outcome of the disease. This strongly suggests that HCMV infection contributes to development and pathogenesis of glioblastoma. Consequently, HCMV is believed to have oncomodulatory properties in case of glioblastoma. Our goal is to study association of HCMV IE1 protein with chromosomes in glioblastoma cells. We plan to study the functional significance of the novel IE1 localization pattern. We believe that unravelling the function of IE1 specific localization will not only further our understanding of the role that HCMV plays in glioblastoma, but may aid in future development of new therapeutic approaches targeting viral persistence.
In our research apart from classical virology methods (eg. virus production, infectivity assay) we use mostly molecular biology (eg. cloning, EMSA, Southern blot) and cell biology (eg. immunofluorescent staining, FISH, chromosome spreads) methods.
Grants funding our research:
- SONATA BIS 7 from National Science Centre, grant number: 2017/26/E/NZ6/01124, funding period: 2018 – 2023, title: „Exploring mechanisms of congenital HCMV infection: replication, spread and latency establishment”,
- OPUS24 from National Science Centre, grant number: 2022/47/B/NZ6/02446, funding period: 2023 – 2027, title: “Painting or Spots? – Unravelling the Mechanism of Formation and the Functional Significance of the Novel, Glioblastoma-Specific Localization Pattern of HCMV (Human Cytomegalovirus) IE1 (immediate early 1) Protein”.



